- Home
- Live Blog
- Breaking News
- Top Headlines
- Cities
- NE News
- Sentinel Media
- Sports
- Education
- Jobs

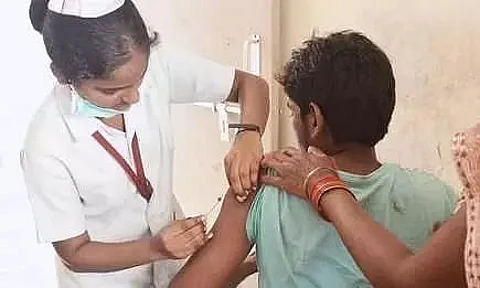
Moradabad, Uttar Pradesh: With India's mass drive on COVID-19 vaccination, the death of a ward boy in Uttar Pradesh's Moradabad has questioned the credibility of the vaccines approved by the Centre. According to reports, a 46-year-old ward boy from a district hospital in Moradabad died within 24 hours after receiving the covishield coronavirus vaccine.
The deceased identified as Mahipal Singh reportedly complained of breathing problem and unease in the chest after receiving a dose of Covishield, one of the recommended vaccine by the Ministry of Health.
Report quoted Dr MC Garg, the chief medical officer (CMO) of the district hospital, saying- "Mahipal Singh (the ward boy) was given the Covishield vaccine at about 12 noon on Saturday."
However, a day later, he suffered pain in the chest with breathlessness. Meanwhile, hospital authority said that the ward boy did not work the night shift after vaccination.
"The death may not be due to any side effect of the vaccine. We are trying to verify the exact reason for his death, "the chief medical officer (CMO) of the hospital was quoted as saying by TOI.
The Covishield vaccine has been prepared by the Serum Institute of India (SII).
On the other hand, on January 16, COVAXIN which is among the two vaccines which got approval for emergency use in the country said that they will compensate if the vaccine causes any side effects or adverse effects to the recipients.
The central government has given an order of 55 lakh doses of Covaxin to the manufacturer. The manufacturer, Bharat Biotech said in a consent form, "In case of any adverse events or serious adverse events, you will be provided with a medically recognised standard of care in the government designated and authorised centres/hospitals. The compensation for the serious adverse event will be paid by the sponsor (BBIL) in case if the SAE is proven to be causally related to the vaccine."
The form will be signed by the vaccine recipients. During Phase 1 and Phase 2 trials, the vaccine has shown the ability to produce antidotes against the deadly coronavirus.
Concerned about the Covaxin vaccine's lack of complete trial, the Resident Doctors' Association (RDA) of the Ram Manohar Lohia Hospital, Delhi, requested the medical superintendent to vaccinate them with Covishield vaccine and not Covaxin, through a letter.
A total of 2,24,301 beneficiaries (2,07,229 on day one) vaccinated till 17th January as per the provisional reports, according to the union health ministry.