- Home
- Live Blog
- Breaking News
- Top Headlines
- Cities
- NE News
- Sentinel Media
- Sports
- Education
- Jobs

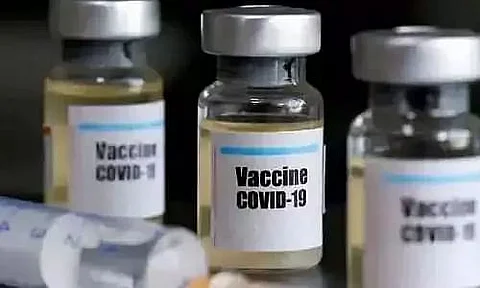
KATHMANDU, Jan 15: Ahead of signing a major COVID vaccine deal with India, probably on Friday, Nepal's Department of Drug Administration (DDA) has given permission for the emergency use of India-made coronavirus vaccine Covishield in the country.
Nepal's Foreign Minister Pradeep Gyawali is currently in New Delhi to take part in the sixth meeting of Nepal-India Joint Commission. One of the major agreements that both sides are likely to ink is on cooperation in COVID vaccine manufactured in India.
The vaccine, developed by the Oxford University and Astra-Zeneca and produced by the Serum Institute of India, has been given conditional permission for emergency use, the department said. The DDA had two days ago called for the registration of vaccines to be used for emergency use.
This is the first time that any COVID-19 vaccine has been approved for use in Nepal. Other manufacturing countries like China and Russia were also interested in providing vaccines to Nepal but due to climatic conditions, pricing, logistic facility and ease of import, Nepal preferred the Indian COVID vaccine.
India has already permitted the use of Covishield as well as Covaxin of Bharat Biotech. (IANS)
Also Watch Adivasis of Assam Demand ST Status